Past, present and future:
using tumour evolution to improve prevention, diagnosis and treatment of cancer
What happens when a cancer seems to have responded well to therapy but then keeps coming back, evolving to be more lethal and evading treatment options? With the number of people successfully treated for their initial cancer steadily increasing, the question of how cancers evolve has become central to cancer research – and discovering how to apply the latest technologies to outmaneuver it is one of the largest challenges for cancer researchers and clinicians today.
OICR launched its Adaptive Oncology initiative this year to bring researchers together to develop and make use of new technologies that can track the evolution of tumours inside the body and learn how cancer changes over time, evading both the body’s immune system and treatments.

Dr. Lincoln Stein
It’s a new approach that can be likened to a long and high stakes chess game. If clinicians are always able to predict the cancer’s next move then it cannot win. However, predicting these moves requires having more than one ‘set of eyes’ on the board. Genomics, pathology, imaging and other disciplines need to be used together to get a full understanding of the path an individual tumour will likely take. The Adaptive Oncology team is working together to develop the technologies needed to predict cancer’s moves and stop it in its tracks – ultimately helping to prevent patients’ cancers from ever becoming fatal.
“The concept of tumour evolution has long interested scientists, but it was not until very recently that the technologies became available to take a deep look at tumours over time and understand how the mechanisms of evolution work,” explains Dr. Lincoln Stein, Head, Adaptive Oncology. “Our belief is that once we can better understand the tumour’s ‘game plan’ – how it is evolving in space and in time - then we can then use that information to develop strategies that try to head off its evolutionary changes.”
New method enables tumour sequencing using a simple blood sample
Understanding a tumour’s game plan begins at the genomic level, where studying how it evolves in a patient can help to better characterize the cancer and better predict the tumour’s next move. But following a tumour’s evolution within a patient requires periodic genomic sequencing so that changes can be tracked over time, in order to know whether the tumour is becoming more aggressive or whether a treated cancer has returned. This requires collecting multiple biopsies from the patient in order to get individual samples for sequencing, which is traditionally an uncomfortable experience that patients often try to avoid. Adaptive Oncology researchers are developing methods to obtain the necessary samples for sequencing in less invasive ways than a standard biopsy, improving patient quality of life and paving the way for broader clinical uptake of adaptive oncology strategies.
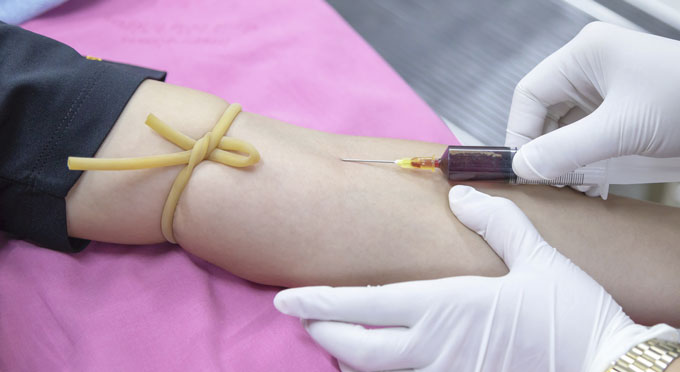
Massive technical leaps in genomic sequencing over the last decade now make it possible to sequence vanishingly small amounts of tumour DNA. That, in turn, has made it possible in some cases to use a simple blood sample, known as a liquid biopsy, to sequence tumours using cell-free DNA present in the bloodstream. This means patients only need to provide blood samples to their clinician instead of undergoing an invasive biopsy. Being able to surveil cancer in this way has opened up the door to the more widespread use of precision medicine – a treatment strategy centred on using the specifics of a patient’s tumour to select therapies – and will help in developing new strategies to track and treat cancer before it becomes fatal.
Another set of technologies allows researchers to sequence the genomes of single cell. Using these tools, OICR researchers can profile the precise mixture of normal, cancerous, and reacting host cells in a tumour, and study how the mixture changes over time.
“Genomic sequencing has been applied in the context of precision medicine with mixed success. The heterogeneity of tumours means that even if a druggable mutation is discovered in a patient’s cancer it may only be present in a portion of the tumour’s cells, meaning the rest of the tumour cells aren’t properly treated,” says Stein. “We’re working to improve genomic sequencing to detect more of the mutations contained in a tumour using technologies such as single-cell sequencing. This will give us a more complete picture of the complexity of a patient’s tumour and, as a result, a better idea of what treatment options to pursue.”
Precision imaging reveals another layer of tumour evolution
The advanced genomic sequencing techniques employed by Adaptive Oncology researchers are an important plank of the initiative, but provide only one lens in which to view a tumour. “Sequencing gives us the genetic information we need about a tumour, but you can only get the ‘big picture’ if you can also track its evolution at the structural and anatomical levels as well,” says Stein.
Dr. Aaron Fenster, Co-Director, of the Adaptive Oncology Imaging Program, is developing robotic instruments to accurately guide fine biopsy needles using ultrasound imaging. The advantage of this approach is that it allows for the precise sampling of different areas of the tumour based upon its appearance, helping to enable the group’s research. The Adaptive Oncology team is looking at whether there is a connection between variation in a tumour’s appearance and genomic heterogeneity.
Sequencing gives us the genetic information we need about a tumour, but you can only get the ‘big picture’ if you can also track its evolution at the structural and anatomical levels as well.
- Dr. Lincoln Stein
“The thinking is that this will improve the results of genomic sequencing downstream by providing a more complete picture of the tumour within the samples that are obtained. We think this will give us a much better chance of identifying all of the mutations that are behind a cancer, which will help clinicians choose the best treatment,” says Fenster. Another benefit to this technology, being developed at the Robarts Research Institute in London, is that it can be used with the types of ultrasound systems commonly found in hospitals, thereby reducing cost and other barriers to clinical adoption.
Fenster’s counterpart as Co-Director of OICR’s Imaging Program is Dr. Martin Yaffe, who is based at the Sunnybrook Research Institute in Toronto. There he is working to develop novel molecular probes for imaging different types of cancer. These probes are designed to enable the detection of subsets of cancer cells and together with information from imaging techniques such as MRI, can be used inform the selection of therapies targeted at these cells and to monitor their effectiveness.
“Imaging tumours to look at their spatial heterogeneity is a relatively novel concept. In the past we did not have the same tools as we do today to detect differences within a tumour with as much detail as we do now. Our group and our collaborators in the Adaptive Oncology initiative are working to understand how these differences in space, looked at over time, explain why some cancers return following treatment and some never fully respond in the first place,” explains Yaffe.
New frontiers in pathology: Looking at cancer cell-by-cell
The improvements in imaging and genome technologies hailed by Fenster, Stein and Yaffe are a huge part of what is making today’s revolution in cancer care and research possible. However, one of the most astonishing advances may be in a field that has been around for hundreds of years – pathology.
Dr. John Bartlett, Director of OICR’s Diagnostic Development program, explains how pathology in cancer is changing and how this transformation plays into Adaptive Oncology.
“Right now, pathologists look at cells through a microscope to see how far they have deviated from a normal appearance. Using his or her experience and pattern recognition, the pathologist will determine how disorganized the tissue is and assign the cancer a grade based upon that. A problem with conventional methods is that a low-grade cancer will look a lot like normal cells,” says Bartlett. While he says that conventional pathology will remain essential to diagnosis for decades to come, it will be augmented by new techniques currently under development by his group and others that will make diagnosis far more accurate and precise.

“What we are doing is taking conventional pathology and putting an extra layer on top. Using computer image analysis, it is now possible to look at and generate data on the length, width, staining intensity, shape and other features of each individual cell in a cancer. It is the same process but we are now using computers to help us look.”
Using data on tens of millions of cancer cells from thousands of patients, Bartlett and his team are looking for features in size and shape that may help more accurately inform an interpretation of a sample by a traditional pathologist. In addition, the group is able to stain cells in a sample to look for multiple specific proteins. For example, a breast cancer sample could be stained to highlight cells that are expressing the estrogen receptor, the progesterone receptor, the HER2 oncogene and a marker of proliferation called KI67.
What we are doing is taking conventional pathology and putting an extra layer on top. Using computer image analysis, it is now possible to look at and generate data on the length, width, staining intensity, shape and other features of each individual cell in a cancer.
- Dr. John Bartlett
“Bringing pathology technologies up to date has allowed us to actually interrogate cancers in a whole new dimension, looking at size and shape and the integrated cell-by-cell expression of proteins in a way we never have before,” says Bartlett. “In a clinical environment, being able to understand these cancers on a cell-by-cell basis, and see the differences within a tumour would provide invaluable information in the selection of treatment and the prevention of relapses by monitoring patients.”
Finding the hallmarks of early cancer
Being able to understand the evolution of tumours is not only valuable information for guiding treatment, but also can help enable the early detection of tumours when they are small and treatable. By using novel sequencing technologies to ‘look back in time’ at the origins and first mutations in tumours, Adaptive Oncology researchers are searching for the genetic hallmarks of early cancer formation.
“One of the ways we are identifying the features associated with early cancer formation is by studying the thousands of blood samples provided by participants in the Ontario Health Study,” says Stein. “We are sequencing these samples and then cross referencing those results with health records and other data to see if there are common early evolutionary changes in those people who have developed cancer.”
Discovering these molecular features can enable the development of new ways to identify those at risk of cancer later in life. These individuals can then be directed to appropriate screening programs so that if cancer does begin to form it can be treated in its earliest stages.
Better understanding cancer to improve prevention, diagnosis and care
As researchers unveil the complexities of cancer cells, it is becoming increasingly necessary to address research questions with expertise from different disciplines of oncology. The Adaptive Oncology initiative harnesses Canada’s rich cohort datasets, OICR’s liquid biopsy technologies, and Ontario’s expertise in precision imaging to make breakthroughs in cancer research – ones that are ready to adapt and stay one step ahead of the disease.
“It’s thrilling to be conducting research at a time of such a transformational change in technology and to be at the forefront of bring the benefits of these tools to patients in Ontario and around the world,” says Stein. “We are proud to have brought together some of the province’s best cancer scientists to put the concept of adaptive oncology into practice. Through our multidisciplinary approach we are working to produce true innovations that will help many patients live long, healthy lives beyond their initial diagnosis.”